72. One-step aerosol synthesis of KBaTeBiO6 for CO2 photoreduction
Hao Zhou, et al. Nanoscale (2021)
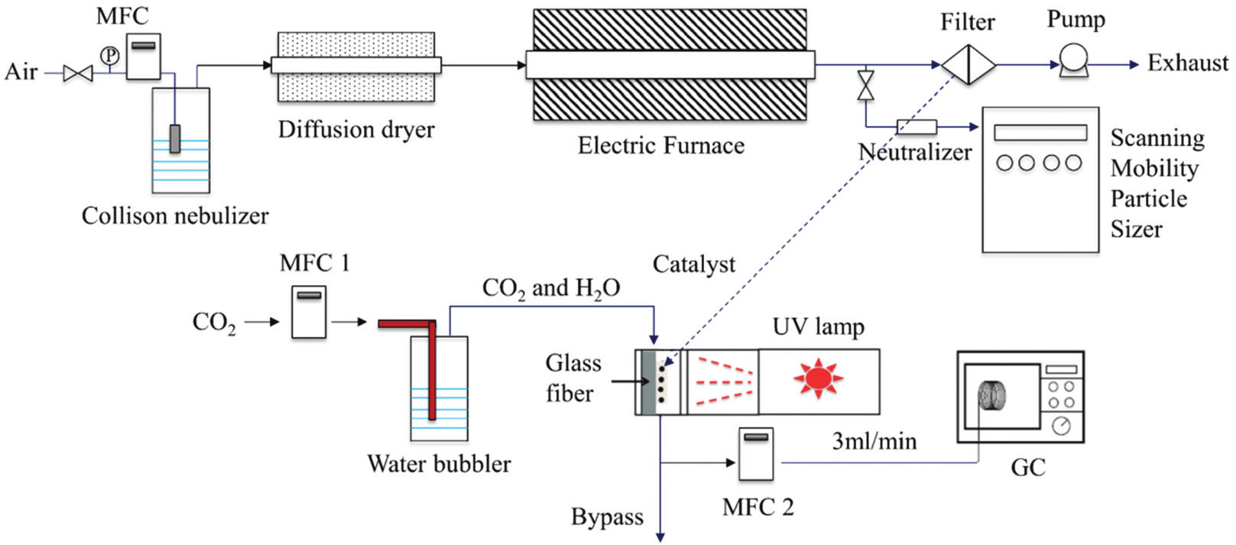
One-step aerosol synthesis of a double perovskite oxide (KBaTeBiO6) as a potential catalyst for CO2 photoreduction
Hao Zhou,Mojgan Kouhnavard,Sungyoon Jung,Rohan Mishra and Pratim Biswas, Nanoscale (2021)
This study presents a comprehensive investigation on the aerosol synthesis of a semiconducting double perovskite oxide with a nominal composition of KBaTeBiO6, which is considered as a potential candidate for CO2 photoreduction. We demonstrate the rapid synthesis of the multispecies compound KBaTeBiO6 with extremely high purity and controllable size through a single-step furnace aerosol reactor (FuAR) process. The formation mechanism of the perovskite through the aerosol route is investigated using thermogravimetric analysis to identify the optimal reference temperature, residence time and other operational parameters in the FuAR synthesis process to obtain highly pure KBaTeBiO6 nanoparticles. It is observed that particle formation in the FuAR is based on a combination of gas-to-particle and liquid-to-particle mechanisms. The phase purity of the perovskite nanoparticles depends on the ratio of the residence time and the reaction time. The particle size is strongly affected by the precursor concentration, residence time and furnace temperature. Finally, the photocatalytic performance of the synthesized KBaTeBiO6 nanoparticles is investigated for CO2 photoreduction under UV-light. The best performing sample exhibits an average CO production rate of 180 μmol g−1 h−1 in the first half hour with a quantum efficiency of 1.19%, demonstrating KBaTeBiO6 as a promising photocatalyst for CO2 photoreduction.