43. Sharp Transitions in Single Lead Bromide Nanocrystals
Yin et al., J. Mater. Chem. C (2019)
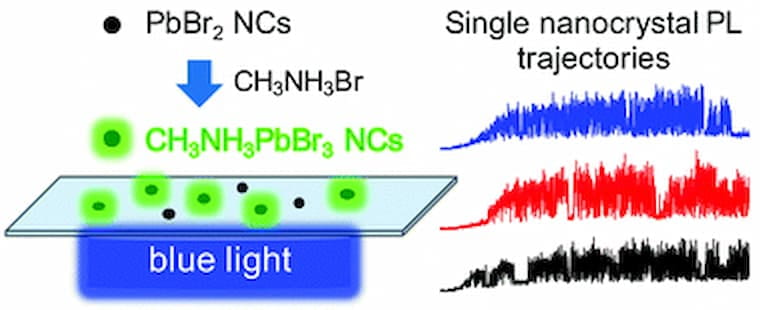
Fluorescence microscopy of single lead bromide nanocrystals reveals sharp transitions during their transformation to methylammonium lead bromide
Bo Yin, John Cavin, Dong Wang, Daniel Khan, Meikun Shen, Craig Laing, Rohan Mishra, Bryce Sadtler
Journal of Materials Chemistry C (2019)
Control over the nucleation and growth of lead-halide perovskite crystals is critical to obtain semiconductor films with high quantum yields in optoelectronic devices. In this report, we use the change in fluorescence brightness to image the transformation of individual lead bromide (PbBr2) nanocrystals to methylammonium lead bromide (CH3NH3PbBr3) via intercalation of CH3NH3Br. Analyzing this reaction one nanocrystal at a time reveals information that is masked when the fluorescence intensity is averaged over many particles. Sharp rises in the intensity of single nanocrystals indicate they transform much faster than the time it takes for the ensemble average to transform. While the ensemble reaction rate increases with increasing CH3NH3Br concentration, the intensity rises for individual nanocrystals are insensitive to the CH3NH3Br concentration. To explain these observations, we propose a phase-transformation model in which the reconstructive transitions necessary to convert a PbBr2 nanocrystal into CH3NH3PbBr3 initially create a high energy barrier for ion intercalation. A critical point in the transformation occurs when the crystal adopts the perovskite phase, at which point the activation energy for further ion intercalation becomes progressively smaller. Monte Carlo simulations that incorporate this change in activation barrier into the likelihood of reaction events reproduce key experimental observations for the intensity trajectories of individual particles. The insights gained from this study may be used to further control the crystallization of CH3NH3PbBr3 and other solution-processed semiconductors.