85. A Method for Predicting the Phase Stability of Alloys using Pairwise Mixing Enthalpy
Zhaohan Zhang et al., Acta Materialia (2022)
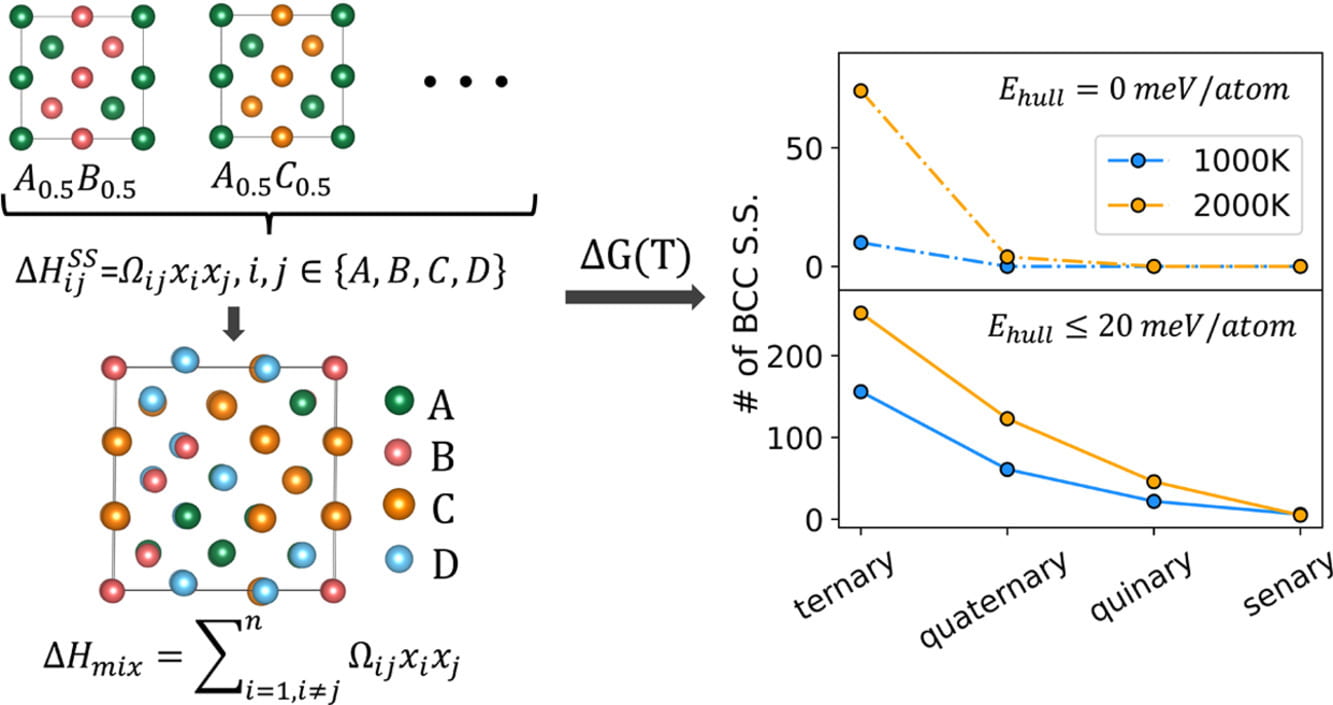
A Fast and Robust Method for Predicting the Phase Stability of Refractory Complex Concentrated Alloys using Pairwise Mixing Enthalpy
Zhaohan Zhang, Mu Li, John Cavin, Katharine Flores and Rohan Mishra
The ability to predict the composition- and temperature-dependent stability of refractory complex concentrated alloys (RCCAs) is vital to the design of high-temperature structural alloys. Here, we present a model based on first-principles calculations to predict the thermodynamic stability of multicomponent equimolar solid solutions in a high-throughput manner and apply it to screen over 20,000 compositions. We develop a database that contains pairwise mixing enthalpy of 17 refractory metals using density-functional theory (DFT)-based total energy calculations. To these, we fit thermodynamic solution models that can accurately capture the mixing enthalpy of multicomponent BCC solid solutions. By comparing their energy with DFT-calculated enthalpy of intermetallics from the Materials Project database and using convex hull analyses, we identify the stable phase of any RCCA as a function of temperature. The predicted stability of NbTiZr, NbTiZrV, and NbTiZrVM (M = Mo,Ta,Cr) systems as a function of temperature agree well with prior experimental observations. We apply our model to predict the phase evolution in NbVZr-Tix (0 < x < 1), which is confirmed experimentally using a high-throughput, laser deposition-based synthesis technique. This method provides a fast and accurate way to estimate the phase stability of new RCCAs to expedite their experimental discovery.