52. Role of Solid-State Miscibility in Cesium Lead Halide
Wang et al., J. Phys. Chem. Letters (2020)
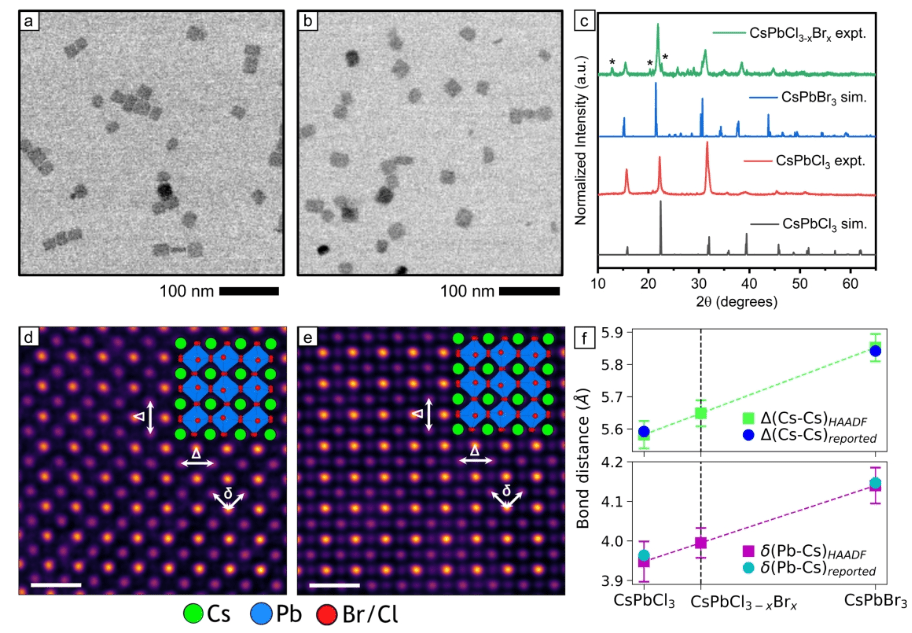
Role of Solid-State Miscibility during Anion Exchange in Cesium Lead Halide Nanocrystals Probed by Single-Particle Fluorescence
Dong Wang, John Cavin, Bo Yin, Arashdeep Singh SinghThind, Albina Y. Borisevich, Rohan Mishra, and Bryce Sadtler Journal of Physical Chemistry Letters (2020)
In this Letter, we used fluorescence microscopy to image the reversible transformation of individual CsPbCl3 nanocrystals to CsPbBr3, which enables us to quantify heterogeneity in reactivity among hundreds of nanocrystals prepared within the same batch. We observed a wide distribution of waiting times for individual nanocrystals to react as has been seen previously for cation exchange and ion intercalation. However, a significant difference for this reaction is that the switching times for changes in fluorescence intensity are dependent on the concentration of substitutional halide ions in solution (i.e., Br– or Cl–). Based on the high solid-state miscibility between CsPbCl3 and CsPbBr3, we develop a model in which the activation energy for anion exchange depends on the density of exchanged ions in the nanocrystal. The heterogeneity in reaction kinetics observed among individual nanocrystals limits the compositional uniformity that can be achieved in luminescent CsPbCl3-xBrx nanocrystals prepared by anion exchange.